Parkinson’s Disease, Freezing of Gait and Walking Automaticity
Many walking abnormalities in people with Parkinson’s disease may be characterized by a shift in locomotor control from healthy automaticity by basal ganglia to compensatory executive control by the cerebral cortex (1). This shift to less automaticity is potentially detrimental to walking performance as executive control strategies are not optimized for locomotor control, place excessive demands on a limited cognitive reserve, and continuously require attention. Traditionally, when gait slows or becomes more variable because of performing another task while walking (dual-task), control of gait is assumed to be less automatic. However, direct physiological biomarkers of gait automaticity have been limited. Recent development of wireless, functional near infrared spectroscopy (fNIRS) provides more direct, physiological measures of automaticity via mobile imaging of cortical blood flow reflecting neural activity.
The ability to quantitatively and directly assess whether a patient requires higher-level cognitive processes to stand and walk is important because functional mobility in real world environments requires safe, automatic control of balance and gait while simultaneously attending to relevant stimuli (e.g., traffic signs) and/or distracting stimuli (e.g., baby’s cry) with higher level cognitive processes. If walking is not controlled automatically by subcortical areas, the attention networks involving the prefrontal cortex (PFC) may fail to pay attention to walking or balancing, even momentarily, leading to devastating falls.
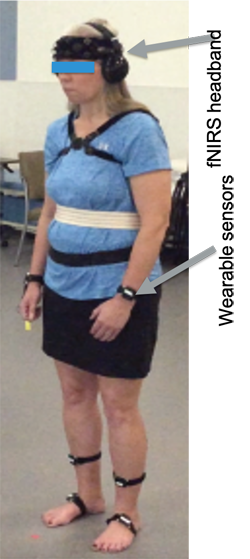
Figure 1: Study participant with fNIRS headband and wearable sensors.
In our laboratory, we simultaneously measured prefrontal cortex activity and mobility characteristics, during walking and turning. Participants walked, at self-selected comfortable pace, back and forth over a 9 meter straight path, with a 180° turn at each end and performed also 360 degrees turning in place. Two conditions were tested: single and dual-task walking and turning in place. Each condition included an initial 20 s of quiet standing (baseline period) followed by 80 s of walking or turning (task period). The dual-task condition consisted of combining the walking/turning task with a concurrent, sustained attention task (auditory Modified AX-Continuous Performance Task), which required participants to press a handheld button after a two-paired letters sequence. Figure 1 shows the set-up with the fNIRS headband to quantify PFC activity and wearable sensors to quantify gait and turning.
Our work (2-4) and the work of others (5-7) recently showed that people with PD have less automatic walking and turning, as evidenced by more activity in the PFC, measured by mobile imaging with fNIRS.
Figure 2: PFC HbO2 levels in healthy controls and people with PD during walking in single-task and dual-task.
Fig.2 shows the larger PFC activity in the PD group compared to the control group during single task walking (purple), but during dual-task walking (gray). Healthy controls but not participants with PD showed an additional increase in PFC activity during dual-task walking, compared to single-task walking, suggesting that PD results in maximal use of PFC to control even single-task walking (3). Such finding is in line with results from other studies (5,8,9).
Our work also showed that automaticity of walking and turning is even more affected in people with PD who experience Freezing of Gait (FoG) compared to those who do not have freezing (2,10). FoG is one of the most debilitating walking impairments in PD, defined as a “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk. It is possible that people with PD experiencing FoG require the allocation of additional prefrontal executive resources for the control of walking, which is likely a compensatory mechanism to maintain gait performance comparable to non-freezers. Interestingly, spatio-temporal gait parameters were similar in people with and without FoG, but deteriorated in the dual-task, compared to single-task, condition. Since PFC activity was similar between single-task and dual-task walking, it is possible that participants allocated most of their available prefrontal cognitive resources for the performance of the cognitive task in the dual-task condition. As a consequence, gait deteriorated because it lacked the required level of prefrontal cognitive resources.
Our findings suggest that PFC should be targeted to develop effective interventions aiming to improve gait and turning in people with PD. Future studies using fNIRS measures of PFC activity could help in understanding whether rehabilitation interventions improve automaticity of walking, as well as characteristics of walking.
References
1 Wu, T., Hallett, M. & Chan, P. Motor automaticity in Parkinson's disease. Neurobiol Dis 82, 226-234, doi:10.1016/j.nbd.2015.06.014 (2015).
2 Belluscio, V., Stuart, S., Bergamini, E., Vannozzi, G. & Mancini, M. The Association between Prefrontal Cortex Activity and Turning Behavior in People with and without Freezing of Gait. Neuroscience 416, 168-176, doi:10.1016/j.neuroscience.2019.07.024 (2019).
3 Stuart, S., Belluscio, V., Quinn, J. F. & Mancini, M. Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson's Disease. Frontiers in neurology 10, 536, doi:10.3389/fneur.2019.00536 (2019).
4 Stuart, S. & Mancini, M. Prefrontal Cortical Activation With Open and Closed-Loop Tactile Cueing When Walking and Turning in Parkinson Disease: A Pilot Study. Journal of neurologic physical therapy : JNPT 44, 121-131, doi:10.1097/npt.0000000000000286 (2020).
5 Maidan, I. et al. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson's Disease and Healthy Older Adults: An fNIRS Study. Neurorehabilitation and neural repair 30, 963-971, doi:10.1177/1545968316650426 (2016).
6 Maidan, I. et al. Altered brain activation in complex walking conditions in patients with Parkinson's disease. Parkinsonism & related disorders 25, 91-96, doi:10.1016/j.parkreldis.2016.01.025 (2016).
7 Nieuwhof, F. et al. Measuring prefrontal cortical activity during dual task walking in patients with Parkinson's disease: feasibility of using a new portable fNIRS device. Pilot Feasibility Stud 2, 59, doi:10.1186/s40814-016-0099-2 (2016).
8 Gramigna, V. et al. Near-Infrared Spectroscopy in Gait Disorders: Is It Time to Begin? Neurorehabilitation and neural repair 31, 402-412, doi:10.1177/1545968317693304 (2017).
9 Maidan, I., Bernad-Elazari, H., Giladi, N., Hausdorff, J. M. & Mirelman, A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson's Disease? Brain topography 30, 531-538, doi:10.1007/s10548-017-0564-0 (2017).
10 Vitorio, R., Stuart, S. & Mancini, M. Executive Control of Walking in People With Parkinson's Disease With Freezing of Gait. Neurorehabilitation and neural repair34, 1138-1149, doi:10.1177/1545968320969940 (2020)
Martina Mancini, PhD is Assistant Professor of Neurology and Co-director of the Balance Disorders Laboratory in the Parkinson’s Center of Oregon at Oregon Health & Science University, Portland, Oregon. The Balance Disorders Laboratory is on the forefront of developing and implementing objective measures to quantify balance deficits in people with Parkinson’s disease (PD). Although we found that objective measures in the clinic or laboratory are very reliable and sensitive to subtle balance impairments, quantification of prescribed balance and gait tasks does not adequately reflect typical mobility function during daily life. In addition to studying how balance control is disrupted, our laboratory develops novel balance training interventions and uses state-of-the-art brain imaging (e.g. MRI, EEG, fNIRS) to determine the neural correlates of balance impairments and rehabilitation efficacy in people with PD. Professor Mancini spoke at the WPC Virtual Congress in 2021 on Innovative Approaches to Reduce Freezig of Gait. Watch her talk HERE.
Ideas and opinions expressed in this post reflect that of the author solely. They do not reflect the opinions or positions of the World Parkinson Coalition®